Cases gear up for Summer 2015 trials
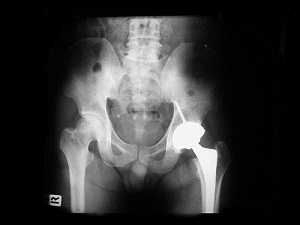
With more than 600 Stryker Rejuvenate hip cases centralized as part of an MDL in the federal court system [more on the MDL process], U.S. District Court Judge Donovan Frank has indicated that a small group of cases will be ready for trial in Summer of 2015.
This small group of cases, known as “bellwether” cases, are scheduled for early trials designed to get some preliminary data on how juries react to the cases, so that the parties can make informed decisions on settlement. This is crucial towards moving the litigation forward and ultimately towards resolution. Cases don’t settle because the parties disagree on the value of the claims.
Trying a few cases helps everyone take an honest look at the merits and the value of the cases. Practically, many medical device companies have in-house counsel that tries to brainwash the real people making the decisions that plaintiffs’ claims can be defended. Then you have the defense lawyers who are making plenty of money and have every interest in seeing the cases go on. I think if the Stryker higher-ups see a few verdicts, they will stop listening to their in-house counsel and their outside lawyers because the juries will be speaking loud and clear.
So the only really bad news for plaintiffs is that the trials don’t begin until 2015. There is no arguing with the fact that justice is painfully slow in these cases.
In addition to those cases filed in federal court, there are at least 873 cases pending in New Jersey state court, with additional claims filed in Florida state court. In New Jersey, all of the Stryker hip lawsuits have been centralized before one Superior Court judge as part of a Multi-County Litigation (MCL). MCLs consolidate the cases for discovery purposes, as there are similarities between the underlying cases such as witnesses and evidence that will be common to all of these Stryker hip lawsuits.
Last year, a mediation process was established in an attempt to see if the Stryker Rejuvenate cases could be settled. At least eight of the nine cases that have gone to mediation have agreed to settle. Continue reading
 Recently, we told you that there is a lot of research suggesting that testosterone treatments for men can cause an increased risk of heart attacks and strokes, leading to serious injury and death. European drug regulators have jumped on board and have now launched their own investigation into the heart risks associated with low testosterone drugs.
Recently, we told you that there is a lot of research suggesting that testosterone treatments for men can cause an increased risk of heart attacks and strokes, leading to serious injury and death. European drug regulators have jumped on board and have now launched their own investigation into the heart risks associated with low testosterone drugs. Lawsuit Information Center
Lawsuit Information Center